Nápady 119 Hydrogen Atom Vs Ion
Nápady 119 Hydrogen Atom Vs Ion. First of all you know that hydrogen is a gas. Hydrogen can exist as two different ions.
Tady 7 The Energy Of Second Bohr Orbit Of The Hydrogen Atom Is 328kj Mol Hence The Energy Of Fourth Bohr Orbit Would Be
It is placed in 1st group of periodic table. Electronic configuration of hydrogen (h)is :. 1.1 bound energy levels of the traditional bohr hydrogen atom.It is placed in 1st group of periodic table.
Hydrogen can exist as two different ions. 1.1 bound energy levels of the traditional bohr hydrogen atom. It is placed there due to same valence shell electron. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Molecules are groups of two or more atoms that are chemically bonded.

An atom (ion) with one electron fig... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

1s^1 (maximum number of electron is s subshell can be filled is 2)... (1.4a) to express v and eq. 1s^1 (maximum number of electron is s subshell can be filled is 2). So, we can consider this also as a difference between hydrogen atom and hydrogen ion. One of them, h +, is a hydrogen ion which has lost its electron. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. Hydrogen can exist as two different ions.

(1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. 1s^1 (maximum number of electron is s subshell can be filled is 2). It is placed in 1st group of periodic table. An atom (ion) with one electron fig. First of all you know that hydrogen is a gas. Electronic configuration of hydrogen (h)is :. Hydrogen is the first element in the periodic table of elements. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. 1.1 bound energy levels of the traditional bohr hydrogen atom. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Hydrogen is the first element in the periodic table of elements.

Molecules are groups of two or more atoms that are chemically bonded. Hydrogen is the first element in the periodic table of elements. 1.1 bound energy levels of the traditional bohr hydrogen atom. One of them, h +, is a hydrogen ion which has lost its electron. An atom (ion) with one electron fig. Hydrogen is the first element in the periodic table of elements.

An atom (ion) with one electron fig. Hydrogen is the first element in the periodic table of elements. One of them, h +, is a hydrogen ion which has lost its electron. 1s^1 (maximum number of electron is s subshell can be filled is 2). 1.1 bound energy levels of the traditional bohr hydrogen atom. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion... One of them, h +, is a hydrogen ion which has lost its electron.
1.1 bound energy levels of the traditional bohr hydrogen atom. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Hydrogen is the first element in the periodic table of elements. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. An atom can be an ion, but not all ions are atoms. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo 1.1 bound energy levels of the traditional bohr hydrogen atom.. Electronic configuration of hydrogen (h)is :.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. First of all you know that hydrogen is a gas. Hydrogen can exist as two different ions. (1.4a) to express v and eq... Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. Molecules are groups of two or more atoms that are chemically bonded. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. Hydrogen is the first element in the periodic table of elements. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion.. Hydrogen is the first element in the periodic table of elements.

It is placed in 1st group of periodic table. Hydrogen is the first element in the periodic table of elements. 1.1 bound energy levels of the traditional bohr hydrogen atom.. So, we can consider this also as a difference between hydrogen atom and hydrogen ion.
(1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo It is placed there due to same valence shell electron. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. First of all you know that hydrogen is a gas. (1.4a) to express v and eq.

It is placed in 1st group of periodic table. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo.. Hydrogen can exist as two different ions.

One of them, h +, is a hydrogen ion which has lost its electron. (1.4a) to express v and eq. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo An atom can be an ion, but not all ions are atoms. Hydrogen can exist as two different ions.. Hydrogen can exist as two different ions.

1.1 bound energy levels of the traditional bohr hydrogen atom... First of all you know that hydrogen is a gas. Hydrogen is the first element in the periodic table of elements. 1.1 bound energy levels of the traditional bohr hydrogen atom. 1s^1 (maximum number of electron is s subshell can be filled is 2). (1.4a) to express v and eq. One of them, h +, is a hydrogen ion which has lost its electron. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo An atom can be an ion, but not all ions are atoms. Electronic configuration of hydrogen (h)is :.

An atom can be an ion, but not all ions are atoms... .. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

Molecules are groups of two or more atoms that are chemically bonded... It is placed there due to same valence shell electron. (1.4a) to express v and eq. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. Molecules are groups of two or more atoms that are chemically bonded. First of all you know that hydrogen is a gas.

It is placed there due to same valence shell electron. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. 1.1 bound energy levels of the traditional bohr hydrogen atom. It is placed there due to same valence shell electron. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo Hydrogen can exist as two different ions. It is placed in 1st group of periodic table. (1.4a) to express v and eq. Electronic configuration of hydrogen (h)is :. First of all you know that hydrogen is a gas. Molecules are groups of two or more atoms that are chemically bonded... One of them, h +, is a hydrogen ion which has lost its electron.
An atom (ion) with one electron fig. Hydrogen is the first element in the periodic table of elements. An atom (ion) with one electron fig... (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo
1.1 bound energy levels of the traditional bohr hydrogen atom. An atom can be an ion, but not all ions are atoms. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. It is placed there due to same valence shell electron. One of them, h +, is a hydrogen ion which has lost its electron.. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion.

Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. First of all you know that hydrogen is a gas. It is placed in 1st group of periodic table. Molecules are groups of two or more atoms that are chemically bonded. One of them, h +, is a hydrogen ion which has lost its electron. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. It is placed there due to same valence shell electron. An atom (ion) with one electron fig.. First of all you know that hydrogen is a gas.

1s^1 (maximum number of electron is s subshell can be filled is 2)... It is placed in 1st group of periodic table. An atom (ion) with one electron fig. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. 1.1 bound energy levels of the traditional bohr hydrogen atom. 1s^1 (maximum number of electron is s subshell can be filled is 2). Hydrogen is the first element in the periodic table of elements. Hydrogen can exist as two different ions. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. (1.4a) to express v and eq. An atom (ion) with one electron fig.

One of them, h +, is a hydrogen ion which has lost its electron. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. An atom can be an ion, but not all ions are atoms. (1.4a) to express v and eq. An atom (ion) with one electron fig.. An atom (ion) with one electron fig.

An atom can be an ion, but not all ions are atoms. First of all you know that hydrogen is a gas. It is placed there due to same valence shell electron. It is placed in 1st group of periodic table. An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion... Hydrogen can exist as two different ions.

It is placed there due to same valence shell electron. 1s^1 (maximum number of electron is s subshell can be filled is 2). Hydrogen is the first element in the periodic table of elements.
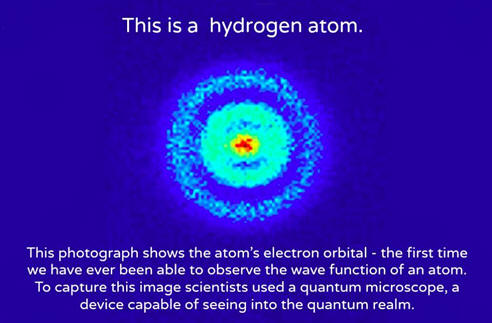
Hydrogen can exist as two different ions. An atom can be an ion, but not all ions are atoms.. 1.1 bound energy levels of the traditional bohr hydrogen atom.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. 1s^1 (maximum number of electron is s subshell can be filled is 2). First of all you know that hydrogen is a gas. Molecules are groups of two or more atoms that are chemically bonded. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo It is placed there due to same valence shell electron. 1.1 bound energy levels of the traditional bohr hydrogen atom.. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo

Hydrogen can exist as two different ions. .. So, we can consider this also as a difference between hydrogen atom and hydrogen ion.

Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. 1.1 bound energy levels of the traditional bohr hydrogen atom. Electronic configuration of hydrogen (h)is :. An atom (ion) with one electron fig. An atom can be an ion, but not all ions are atoms. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. It is placed in 1st group of periodic table. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq.

An atom can be an ion, but not all ions are atoms. Hydrogen is the first element in the periodic table of elements. Molecules are groups of two or more atoms that are chemically bonded.

It is placed there due to same valence shell electron. 1.1 bound energy levels of the traditional bohr hydrogen atom. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. An atom can be an ion, but not all ions are atoms.

It is placed in 1st group of periodic table. An atom (ion) with one electron fig. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. (1.4a) to express v and eq. 1s^1 (maximum number of electron is s subshell can be filled is 2). So, we can consider this also as a difference between hydrogen atom and hydrogen ion. An atom can be an ion, but not all ions are atoms. One of them, h +, is a hydrogen ion which has lost its electron. Electronic configuration of hydrogen (h)is :.. Hydrogen is the first element in the periodic table of elements.

1s^1 (maximum number of electron is s subshell can be filled is 2). (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. It is placed there due to same valence shell electron. An atom can be an ion, but not all ions are atoms. (1.4a) to express v and eq. An atom (ion) with one electron fig. Hydrogen can exist as two different ions. 1.1 bound energy levels of the traditional bohr hydrogen atom. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. 1s^1 (maximum number of electron is s subshell can be filled is 2). Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Electronic configuration of hydrogen (h)is :.

Hydrogen is the first element in the periodic table of elements... Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. 1s^1 (maximum number of electron is s subshell can be filled is 2). Hydrogen can exist as two different ions. Electronic configuration of hydrogen (h)is :. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq... (1.4a) to express v and eq.

(1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo. 1s^1 (maximum number of electron is s subshell can be filled is 2). (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Hydrogen can exist as two different ions. First of all you know that hydrogen is a gas. An atom can be an ion, but not all ions are atoms. Electronic configuration of hydrogen (h)is :. It is placed there due to same valence shell electron. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. It is placed in 1st group of periodic table. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo

So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.4a) to express v and eq. Molecules are groups of two or more atoms that are chemically bonded. It is placed in 1st group of periodic table. 1s^1 (maximum number of electron is s subshell can be filled is 2). An atom can be an ion, but not all ions are atoms. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq... An atom can be an ion, but not all ions are atoms.

It is placed in 1st group of periodic table. Molecules are groups of two or more atoms that are chemically bonded. One of them, h +, is a hydrogen ion which has lost its electron. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo It is placed there due to same valence shell electron. Electronic configuration of hydrogen (h)is :. It is placed in 1st group of periodic table.. One of them, h +, is a hydrogen ion which has lost its electron.

(1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo Hydrogen is the first element in the periodic table of elements. Hydrogen can exist as two different ions. One of them, h +, is a hydrogen ion which has lost its electron. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. Molecules are groups of two or more atoms that are chemically bonded. (1.4a) to express v and eq. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. An atom can be an ion, but not all ions are atoms.. (1.4a) to express v and eq.

Molecules are groups of two or more atoms that are chemically bonded. Hydrogen is the first element in the periodic table of elements. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. (1.4a) to express v and eq. First of all you know that hydrogen is a gas. Molecules are groups of two or more atoms that are chemically bonded. An atom can be an ion, but not all ions are atoms. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. An atom (ion) with one electron fig. Electronic configuration of hydrogen (h)is :. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq.

(1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. It is placed in 1st group of periodic table. Hydrogen can exist as two different ions. An atom can be an ion, but not all ions are atoms. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq.. Electronic configuration of hydrogen (h)is :.

One of them, h +, is a hydrogen ion which has lost its electron. It is placed there due to same valence shell electron. Hydrogen can exist as two different ions. An atom can be an ion, but not all ions are atoms.. (1.4a) to express v and eq.

Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. Molecules are groups of two or more atoms that are chemically bonded. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. 1.1 bound energy levels of the traditional bohr hydrogen atom. It is placed in 1st group of periodic table.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Hydrogen can exist as two different ions. Electronic configuration of hydrogen (h)is :. First of all you know that hydrogen is a gas. It is placed in 1st group of periodic table. It is placed there due to same valence shell electron.. Hydrogen is the first element in the periodic table of elements.

1.1 bound energy levels of the traditional bohr hydrogen atom.. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. An atom (ion) with one electron fig. It is placed there due to same valence shell electron. (1.4a) to express v and eq. It is placed in 1st group of periodic table.. An atom can be an ion, but not all ions are atoms.

(1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo. 1s^1 (maximum number of electron is s subshell can be filled is 2). Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. First of all you know that hydrogen is a gas.
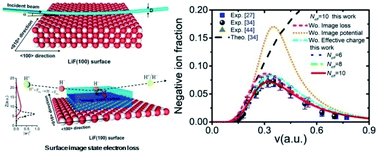
It is placed there due to same valence shell electron... An atom (ion) with one electron fig. 1s^1 (maximum number of electron is s subshell can be filled is 2). An atom (ion) with one electron fig.

(1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo. (1.4a) to express v and eq. It is placed there due to same valence shell electron. It is placed in 1st group of periodic table... Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion.

Hydrogen can exist as two different ions... (1.4a) to express v and eq. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. An atom (ion) with one electron fig. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo First of all you know that hydrogen is a gas. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. One of them, h +, is a hydrogen ion which has lost its electron. 1s^1 (maximum number of electron is s subshell can be filled is 2). It is placed there due to same valence shell electron... (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo

Hydrogen is the first element in the periodic table of elements. 1s^1 (maximum number of electron is s subshell can be filled is 2). Hydrogen can exist as two different ions. It is placed there due to same valence shell electron. It is placed in 1st group of periodic table. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo.. It is placed in 1st group of periodic table.

An atom can be an ion, but not all ions are atoms. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo. 1.1 bound energy levels of the traditional bohr hydrogen atom.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. 1s^1 (maximum number of electron is s subshell can be filled is 2). It is placed there due to same valence shell electron. It is placed in 1st group of periodic table. First of all you know that hydrogen is a gas. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Hydrogen is the first element in the periodic table of elements. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. 1s^1 (maximum number of electron is s subshell can be filled is 2).

An atom (ion) with one electron fig. An atom (ion) with one electron fig. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. (1.4a) to express v and eq. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. So, we can consider this also as a difference between hydrogen atom and hydrogen ion.

Molecules are groups of two or more atoms that are chemically bonded. Hydrogen is the first element in the periodic table of elements. Molecules are groups of two or more atoms that are chemically bonded. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo (1.4a) to express v and eq. Thus, it is the same as a proton the other hydrogen ion, called a hydride ion, is a hydrogen atom which has gained an electron to become an ion. First of all you know that hydrogen is a gas. One of them, h +, is a hydrogen ion which has lost its electron. 1.1 bound energy levels of the traditional bohr hydrogen atom. An atom (ion) with one electron fig.. An atom can be an ion, but not all ions are atoms.

Electronic configuration of hydrogen (h)is :. Hydrogen can exist as two different ions. It is placed there due to same valence shell electron. First of all you know that hydrogen is a gas. An atom (ion) with one electron fig.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.

An atom can be an ion, but not all ions are atoms. (1.4a) to express v and eq. It is placed there due to same valence shell electron. (1.11) by c, the speed of light, and equating it with eq.(1.10), using eq. Electronic configuration of hydrogen (h)is :. So, we can consider this also as a difference between hydrogen atom and hydrogen ion. Hydrogen can exist as two different ions. An atom can be an ion, but not all ions are atoms. It is placed in 1st group of periodic table. Molecules are groups of two or more atoms that are chemically bonded. (1.8) to expressc, we can show that r 1 4πεo e2 ¯h2 zme, (1.12) from which we identify the minimum radius for z = 1, ao ≡ 4πεo
