Ideje 51 Carbon Atom Mass Number Výborně
Ideje 51 Carbon Atom Mass Number Výborně. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Mass numbers of typical isotopes of carbon are 12; Neutron number plus atomic number equals atomic mass number:
Tady Unified Atomic Mass Number Atoms
Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. Then you have 9893 atoms of 12c and 107 atoms of 13c. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.
Mass numbers of typical isotopes of carbon are 12; Mass numbers of typical isotopes of carbon are 12; To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. N+z=a.the difference between the neutron number and the atomic number is known as … May be black atomic structure Assume that you have, say, 10 000 atoms of carbon.

N+z=a.the difference between the neutron number and the atomic number is known as … Assume that you have, say, 10 000 atoms of carbon. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. Atomic number, chemical symbol, and mass number. Then you have 9893 atoms of 12c and 107 atoms of 13c... N+z=a.the difference between the neutron number and the atomic number is known as …

Assume that you have, say, 10 000 atoms of carbon.. Then you have 9893 atoms of 12c and 107 atoms of 13c. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Hexagonal density @ 293 k:. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of.

Then you have 9893 atoms of 12c and 107 atoms of 13c. Hexagonal density @ 293 k:

Assume that you have, say, 10 000 atoms of carbon. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. N+z=a.the difference between the neutron number and the atomic number is known as … If you round the number, you will find the most common isotope of carbon.. May be black atomic structure

Hexagonal density @ 293 k:. N+z=a.the difference between the neutron number and the atomic number is known as … (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams.

Mass numbers of typical isotopes of carbon are 12; N+z=a.the difference between the neutron number and the atomic number is known as … Then you have 9893 atoms of 12c and 107 atoms of 13c. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Assume that you have, say, 10 000 atoms of carbon. Hexagonal density @ 293 k: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. All of those decimals at the end tell us that there is more than one isotope of carbon. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. Atomic number, chemical symbol, and mass number.. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u... (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096;

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. Then you have 9893 atoms of 12c and 107 atoms of 13c. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:

Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. Neutron number plus atomic number equals atomic mass number: All of those decimals at the end tell us that there is more than one isotope of carbon.. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.

If you round the number, you will find the most common isotope of carbon. Atomic number, chemical symbol, and mass number. Assume that you have, say, 10 000 atoms of carbon. All of those decimals at the end tell us that there is more than one isotope of carbon. Mass numbers of typical isotopes of carbon are 12; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Its average atomic mass is 12.11. If you round the number, you will find the most common isotope of carbon. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Hexagonal density @ 293 k: Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Neutron number plus atomic number equals atomic mass number: 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams.

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n... N+z=a.the difference between the neutron number and the atomic number is known as … 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: If you round the number, you will find the most common isotope of carbon. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Mass numbers of typical isotopes of carbon are 12; (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; All of those decimals at the end tell us that there is more than one isotope of carbon. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.

Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Assume that you have, say, 10 000 atoms of carbon. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. If you round the number, you will find the most common isotope of carbon. Mass numbers of typical isotopes of carbon are 12; 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Neutron number plus atomic number equals atomic mass number: Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. N+z=a.the difference between the neutron number and the atomic number is known as …. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:
The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. Atomic number, chemical symbol, and mass number. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Then you have 9893 atoms of 12c and 107 atoms of 13c. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams.

To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms... Mass numbers of typical isotopes of carbon are 12; (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Hexagonal density @ 293 k: 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of.. If you round the number, you will find the most common isotope of carbon.

May be black atomic structure. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. If you round the number, you will find the most common isotope of carbon. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Then you have 9893 atoms of 12c and 107 atoms of 13c. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Mass numbers of typical isotopes of carbon are 12; Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Assume that you have, say, 10 000 atoms of carbon.

Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Then you have 9893 atoms of 12c and 107 atoms of 13c.. N+z=a.the difference between the neutron number and the atomic number is known as …

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:.. Its average atomic mass is 12.11. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. May be black atomic structure The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Atomic number, chemical symbol, and mass number.. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms.

(carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; Its average atomic mass is 12.11. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Mass numbers of typical isotopes of carbon are 12; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: All of those decimals at the end tell us that there is more than one isotope of carbon.

Mass numbers of typical isotopes of carbon are 12;. .. Mass numbers of typical isotopes of carbon are 12;

May be black atomic structure Neutron number plus atomic number equals atomic mass number:.. May be black atomic structure

Atomic number, chemical symbol, and mass number... Mass numbers of typical isotopes of carbon are 12; Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Its average atomic mass is 12.11. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Mass numbers of typical isotopes of carbon are 12; 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. N+z=a.the difference between the neutron number and the atomic number is known as … Atomic number, chemical symbol, and mass number. If you round the number, you will find the most common isotope of carbon. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Mass numbers of typical isotopes of carbon are 12; (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; All of those decimals at the end tell us that there is more than one isotope of carbon. Then you have 9893 atoms of 12c and 107 atoms of 13c. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Hexagonal density @ 293 k: If you round the number, you will find the most common isotope of carbon. Its average atomic mass is 12.11. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams.. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.
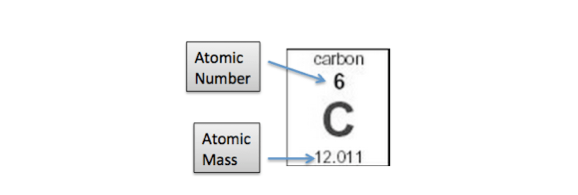
(carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. N+z=a.the difference between the neutron number and the atomic number is known as … Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. If you round the number, you will find the most common isotope of carbon.

Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of... 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. N+z=a.the difference between the neutron number and the atomic number is known as … May be black atomic structure.. N+z=a.the difference between the neutron number and the atomic number is known as …

All of those decimals at the end tell us that there is more than one isotope of carbon. N+z=a.the difference between the neutron number and the atomic number is known as … Neutron number plus atomic number equals atomic mass number: Assume that you have, say, 10 000 atoms of carbon. Hexagonal density @ 293 k: 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:. Then you have 9893 atoms of 12c and 107 atoms of 13c.

(carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. N+z=a.the difference between the neutron number and the atomic number is known as … Mass numbers of typical isotopes of carbon are 12; Assume that you have, say, 10 000 atoms of carbon. Neutron number plus atomic number equals atomic mass number: To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams.

N+z=a.the difference between the neutron number and the atomic number is known as … Then you have 9893 atoms of 12c and 107 atoms of 13c. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Atomic number, chemical symbol, and mass number. If you round the number, you will find the most common isotope of carbon. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: All of those decimals at the end tell us that there is more than one isotope of carbon. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams... If you round the number, you will find the most common isotope of carbon.

Mass numbers of typical isotopes of carbon are 12; Hexagonal density @ 293 k: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:. Assume that you have, say, 10 000 atoms of carbon.

(carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096;. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. N+z=a.the difference between the neutron number and the atomic number is known as … Neutron number plus atomic number equals atomic mass number: Hexagonal density @ 293 k: To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; Mass numbers of typical isotopes of carbon are 12; All of those decimals at the end tell us that there is more than one isotope of carbon.

The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Its average atomic mass is 12.11. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:

N+z=a.the difference between the neutron number and the atomic number is known as … Hexagonal density @ 293 k: Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Neutron number plus atomic number equals atomic mass number: Atomic number, chemical symbol, and mass number. (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; Mass numbers of typical isotopes of carbon are 12; Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of... Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of.

If you round the number, you will find the most common isotope of carbon.. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Then you have 9893 atoms of 12c and 107 atoms of 13c. If you round the number, you will find the most common isotope of carbon.
Neutron number plus atomic number equals atomic mass number: Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Assume that you have, say, 10 000 atoms of carbon. If you round the number, you will find the most common isotope of carbon. Mass numbers of typical isotopes of carbon are 12; 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. N+z=a.the difference between the neutron number and the atomic number is known as … Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of.

(carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096;. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms.

Mass numbers of typical isotopes of carbon are 12; Hexagonal density @ 293 k: The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. Then you have 9893 atoms of 12c and 107 atoms of 13c. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Its average atomic mass is 12.11.

12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: All of those decimals at the end tell us that there is more than one isotope of carbon.. (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096;

3500.0 °c (3773.15 k, 6332.0 °f) boiling point: Then you have 9893 atoms of 12c and 107 atoms of 13c. If you round the number, you will find the most common isotope of carbon. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Neutron number plus atomic number equals atomic mass number: Hexagonal density @ 293 k: Mass numbers of typical isotopes of carbon are 12; The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. N+z=a.the difference between the neutron number and the atomic number is known as …

Then you have 9893 atoms of 12c and 107 atoms of 13c. . 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons:
12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams... The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. Mass numbers of typical isotopes of carbon are 12; 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: N+z=a.the difference between the neutron number and the atomic number is known as … If you round the number, you will find the most common isotope of carbon.

Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively... Then you have 9893 atoms of 12c and 107 atoms of 13c. All of those decimals at the end tell us that there is more than one isotope of carbon. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Neutron number plus atomic number equals atomic mass number: Mass numbers of typical isotopes of carbon are 12; Assume that you have, say, 10 000 atoms of carbon. Mass numbers of typical isotopes of carbon are 12; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:.. All of those decimals at the end tell us that there is more than one isotope of carbon.

Atomic number, chemical symbol, and mass number... Mass numbers of typical isotopes of carbon are 12; 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Assume that you have, say, 10 000 atoms of carbon. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Its average atomic mass is 12.11. Mass numbers of typical isotopes of carbon are 12; May be black atomic structure 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:.. If you round the number, you will find the most common isotope of carbon.

N+z=a.the difference between the neutron number and the atomic number is known as … All of those decimals at the end tell us that there is more than one isotope of carbon. (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Neutron number plus atomic number equals atomic mass number: If you round the number, you will find the most common isotope of carbon. Atomic number, chemical symbol, and mass number. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of.
May be black atomic structure Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. N+z=a.the difference between the neutron number and the atomic number is known as … Hexagonal density @ 293 k: 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively... Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.

3500.0 °c (3773.15 k, 6332.0 °f) boiling point:. Hexagonal density @ 293 k: Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. All of those decimals at the end tell us that there is more than one isotope of carbon... Mass numbers of typical isotopes of carbon are 12;

12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams.. Its average atomic mass is 12.11. Hexagonal density @ 293 k: Neutron number plus atomic number equals atomic mass number:. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms.

Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.. . Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.

Atomic number, chemical symbol, and mass number. May be black atomic structure Atomic number, chemical symbol, and mass number. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. Then you have 9893 atoms of 12c and 107 atoms of 13c. Mass numbers of typical isotopes of carbon are 12;
Atomic number, chemical symbol, and mass number... All of those decimals at the end tell us that there is more than one isotope of carbon. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Then you have 9893 atoms of 12c and 107 atoms of 13c. If you round the number, you will find the most common isotope of carbon. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Assume that you have, say, 10 000 atoms of carbon. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. Atomic number, chemical symbol, and mass number. N+z=a.the difference between the neutron number and the atomic number is known as …

3500.0 °c (3773.15 k, 6332.0 °f) boiling point: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. Mass numbers of typical isotopes of carbon are 12; May be black atomic structure 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: Hexagonal density @ 293 k: Mass numbers of typical isotopes of carbon are 12; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096;

Mass numbers of typical isotopes of carbon are 12; If you round the number, you will find the most common isotope of carbon. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. Neutron number plus atomic number equals atomic mass number: Assume that you have, say, 10 000 atoms of carbon. 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:.. Assume that you have, say, 10 000 atoms of carbon.

12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams... Its average atomic mass is 12.11. Assume that you have, say, 10 000 atoms of carbon. May be black atomic structure. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons:

Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; Atomic number, chemical symbol, and mass number. Hexagonal density @ 293 k: 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: May be black atomic structure 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Chemical state the oxidation state, sometimes referred to as an oxidation number, describes the degree of.. Atomic number, chemical symbol, and mass number.

Assume that you have, say, 10 000 atoms of carbon... All of those decimals at the end tell us that there is more than one isotope of carbon. Atomic number, chemical symbol, and mass number. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:.. Atomic number, chemical symbol, and mass number.

N+z=a.the difference between the neutron number and the atomic number is known as … 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. Hexagonal density @ 293 k: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams... If you round the number, you will find the most common isotope of carbon.

May be black atomic structure. Then you have 9893 atoms of 12c and 107 atoms of 13c. Mass numbers of typical isotopes of carbon are 12; (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; Neutron number plus atomic number equals atomic mass number: 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: All of those decimals at the end tell us that there is more than one isotope of carbon. Its average atomic mass is 12.11.

12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms... 3500.0 °c (3773.15 k, 6332.0 °f) boiling point:

All of those decimals at the end tell us that there is more than one isotope of carbon. Neutron number plus atomic number equals atomic mass number: 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams. Its average atomic mass is 12.11. Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number:

Hexagonal density @ 293 k: May be black atomic structure 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. N+z=a.the difference between the neutron number and the atomic number is known as … Atomic number, chemical symbol, and mass number. Hexagonal density @ 293 k: The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. Mass numbers of typical isotopes of carbon are 12; Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u.. 12 grams / 6.02214129 × 10 23 = 1.9926467 × 10 − 23 grams.

Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Atomic number, chemical symbol, and mass number. 12 × 1.660538921 × 10 − 24 grams = 1.9926467 × 10 − 23 grams. Hexagonal density @ 293 k: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. Mass numbers of typical isotopes of carbon are 12;. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams.
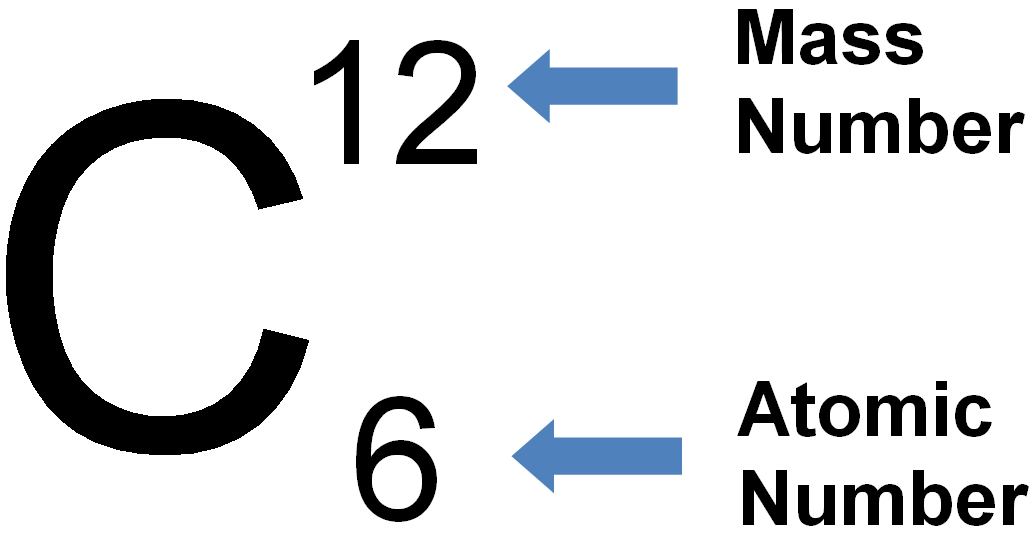
3500.0 °c (3773.15 k, 6332.0 °f) boiling point:.. Then you have 9893 atoms of 12c and 107 atoms of 13c. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; 4827.0 °c (5100.15 k, 8720.6 °f) number of protons/electrons: Atomic number, chemical symbol, and mass number. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n. May be black atomic structure Hexagonal density @ 293 k: The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.

Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Its average atomic mass is 12.11. Neutron number plus atomic number equals atomic mass number: (carbon12)iupac recommend in this case the expression atomic weight, not atomic mass.the atomic weight of carbon (iupac table, 2009) is [12,0096; The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: 3500.0 °c (3773.15 k, 6332.0 °f) boiling point: Then you have 9893 atoms of 12c and 107 atoms of 13c... Its average atomic mass is 12.11.

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Then you have 9893 atoms of 12c and 107 atoms of 13c. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol n.neutron number plus atomic number equals atomic mass number: Mass ofl12c = 9893atoms × 12 u 1atom = 118 716 u. N+z=a.the difference between the neutron number and the atomic number is known as … All of those decimals at the end tell us that there is more than one isotope of carbon. The unified atomic mass unit (u) is 1.660538921 × 10 − 24 grams. Neutron number plus atomic number equals atomic mass number:.. Assume that you have, say, 10 000 atoms of carbon.
